RCH4 - a new ALS drug
At a glance
-
RCH4 is a drug administered by intramuscular injection twice a week. It is administered using a syringe and needle more commonly used to inject insulin in the treatment of diabetes
-
Provided free of charge by this charity (when funding is available) to anyone asking for help, subject to where the patient - the “PALS” lives (A “PALS” is a “Person with ALS” Amyotrophic Lateral Sclerosis, also known as Motor Neurone Disease “MND”), and in accordance with the medical and Regulatory Authority rules relating to prescription drugs in that country. It does not yet have approval
-
PALS who take RCH4: We are not their doctor, and cannot advise them, therefore we refer to them as our “friends”. Nothing on this website may be considered medical advice. PALS must always refer to their doctor or neurologist.
-
Unless there is some clinical contraindication, we accept anyone who asks for help if we have the funds. We have no exclusion criteria. PALS must have a written confirmed diagnosis of ALS or MND, a recent blood plate (test), and have the written permission of a Medical Doctor to take RCH4. There are also some forms that need to be signed, for example, the Patient Informed Consent. There are no related side effects.
-
For 86% of ALS/MND patients, progression is slowed by 65% therefore a ~ threefold increase in life expectancy. No other drug can do this.
-
The evidence is from >80 continual patient-treatment years.
-
For 16% of these subjects, on average it safely stabilizes their condition for years.
-
14% of RCH4 patients are non-responders, i.e. RCH4 has no effect on them.
-
Unfortunately, it is not a cure and no cure exists yet.
-
At this time the exact compounds and how or why they work are currently patented, but not available until a proper investor is found to bring RCH4 through trials and to the global marketplace.
Also, we cannot afford to do blinded, placebo-controlled clinical trials. Accordingly, although there are decades of clinical data indicating notable safety and efficacy over decades of patient-years, in the absence of a 6 months trial (costing $millions) RCH4 must still be considered as an unproven treatment.
Mode of Action
Note: These paragraphs are not intended to be a scientific discussion but a conceptualised broad overview for the average reader.
This new ALS drug targets a newly found agent (“defect”) in the immune system. The fact the the drug, a type of molecule somewhat similar to a monoclonal antibody and reported to be safe, fixes that defect is now certain and undoubted. However, there may other unknown antagonistic agents involved.
The defect is also certainly a factor in the promotion or stimulation of ALS, however it may not be the only defect. There may be others as the aetiology is not clearly understood.
TO REWORD
In essence, the extensive evidence that this new drug which targets dysfunctional lipid rafts is effective in slowing the disease progression across the entire severity spectrum of ALS including both sporadic and familial cases, proving that it targets the primary initiating mechanism of the disease process.
The target
A specific B-cell sub set was identified as the source of the newly recognised agent (operationally termed MRCH) which, in turn, upregulates the expression of a protein, which in excess, is excitotoxic. The B-cell sub set lipid raft receptors were characterised and a molecule developed which recognises that receptor and nothing else - therefore indicating a notably safe Mode Of Action (MOA) profile. This has been confirmed after very many decades of continual-patient-years treatment.
Monthly Report
Patient taking RCH4 are asked to send a report on a monthly basis providing their ALSFRS-R score. A peer reviewed paper confirms that self-evaluation of the ALSFRS-R score concurs almost precisely with the same evaluations done in clinic.
Monthly monitoring reports are submitted to us by all our PALS friends. This monthly information comprises of 6 health parameters. One of these parameters is the ALSFRS-R score. This is an ongoing record of points allocated to 12 questions. As every monthly report form is submitted, the information is automatically picked up by a database and the statistics updated in real time. All interventions known to affect the course of disease (e.g., NIV, Trach., gastrostomy, etc.) are tracked. The monthly monitoring reports also track the drugs and all dietary supplements taken by PALS who are being treated with RCH4.
We therefore maintain a very accurate analysis of each PALS, enabling pro-active ongoing management of their RCH4 treatment if possible.
Efficacy
Based on PALS monthly report we know that:
-
31% of patients exhibit 6 months or more stability
-
52% 4 months or more stability.
-
14% are non responders, i.e., the drug has no effect for them.
It should be noted that although the ALSFRS-R score is the gold standard for decline measurement, it does not include factors such as get-up-and-go, strength etc. It should also be remembered that the ALSFRS-R score may not reflect loss of motor neurons.
However, it is logical and true to observe that, for example, if the ALSFRS-R score does not change, then end of life would be due to something else - or extreme old age.
Efficacy evidence of RCH4 in slowing the progression of ALS, independently reported by PALS themselves, can be seen bellow.
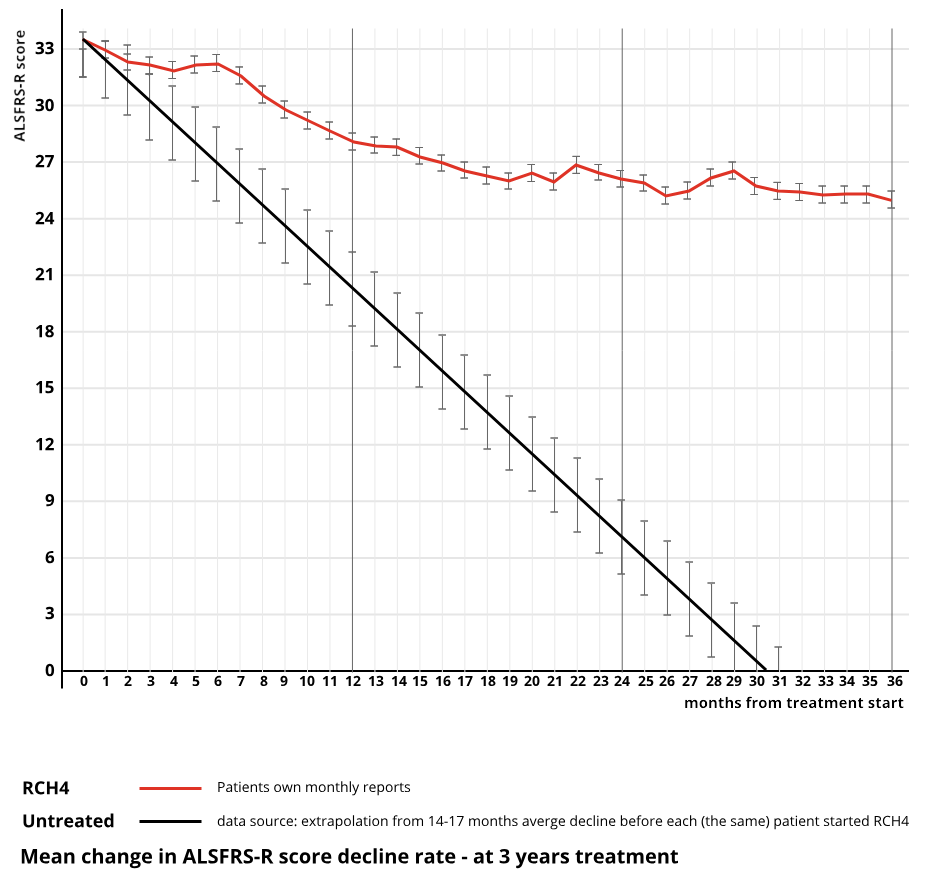
Safety
Although RCH4 has an outstanding safety record and no notable related side effects have ever been reported to date after decades of continual patient-treatment-years, there is always the potential for side effects with any drug.
You would take RCH4 entirely at your own risk.
Cost
There is no cost.
We provide this ALS drug free of charge as a humanitarian imperative.
You are not required to contribute anything. We pay for it ourselves if we have the funds available.
Therefore availability may be intermittent.
Availability
This is an Investigational New ALS Drug which does not have health Regulatory Authority marketing approval in any country. The only legal way to get access to any drug is:
-
If it has Regulatory Authority marketing approval
-
If it is in a clinical trial.
A clinical trial requires Regulatory Authotity permission and a large number of patients. It typically costs (the clinic charges and the drug) Euro 17,000 ($19,000 US) to treat each patient in a trial. We cannot afford to do clinical trials. -
If the Authorities grant permission for compassionate use.
A doctor makes an application for permission to treat the patient on compassionate grounds. This is normally only granted in a case where the patient is very ill and not responding to existing drugs or where there is no effective drug for the disease in question. There is no Regulatory Authority approved drug to cure ALS. -
In accordance with the “right to try” rules.
Most countries have some mechanism to allow a patient to use an experimental new drug. Rules differ and can be very complex and time consuming to deal with. We cannot help in advising about the local regulations in the country where you live. It is for a doctor to decide to allow you to take RCH4. You would need to discuss this with him or her and get written permission. Also, other paperwork is required, e.g. Informed Consent, copies of Neurologists diagnosis etc.
Also, before we start with a new patient, we must be sure that we will have sufficient supply of the drug to reliably provide treatment for the new patient for a long time. Existing PALS must have priority. Accordingly we can only accept small numbers of patients.
If we could, we would supply anyone who would benefit.
There is also the fact that it can take up to 4 months to have each batch manufactured and we must have the funds to pay the Pharmaceutical drug manufacturer in advance. Their minimum production run order keeps our existing PALS friends supplied for over one year. The only way forward to make it permanently available worldwide for everyone, will be the involvement of a Pharmaceutical company or PALS themselves set up their own commercial entity to obtain Regulatory Authority marketing approval for their own medical and financial benefit.
Comparisons with other treatments
At 6 months of treatment
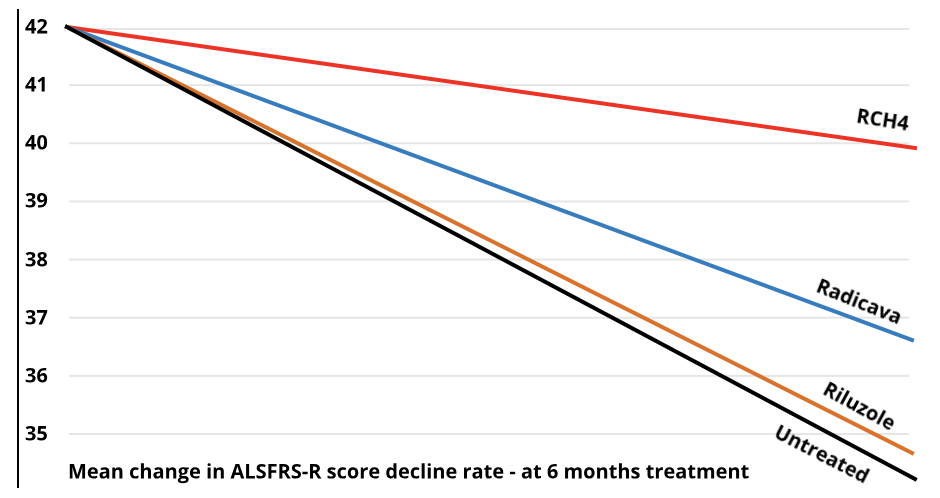
At 3 years of treatment
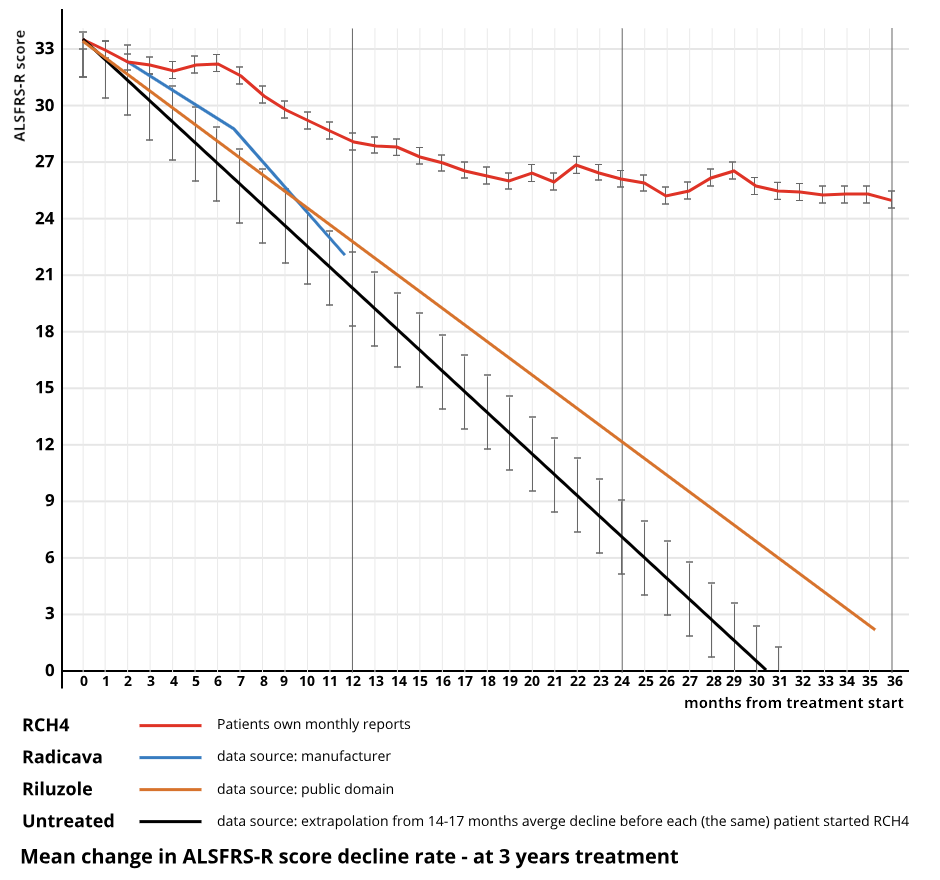
Riluzole™
also known as `Rilutek`
Riluzole™ was originally used in a photographic developing solution, and later as an industrial bleach. It was accidentally screened in an in-vitro assay of glutamate inhibition and found to be slightly effective. The first approved ALS drug `Riluzole`™ (Sanofi Aventis) may not slow the progression rate sufficient to reflect in the ALSFRS-R score. It extends “time to tracheotomy” by 75 days + - 15 days (Source: Sanofi). This data emanates from a very large study of some 900 ALS patients. Extended time to tracheotomy does not necessarily equate to life extension. It does not appear to slow the progression of ALS. Slowing or stopping the progression does equate to life extension - provided that infections or other events are avoided. As far as is known, no ALSFRS-R decline charts have been published for `Riluzole`™ by the manufacturer.
Radicava™
Radicava™ (Mitsubishi Tenabe Japan) (also known as Edaravone) is a form of Pyrazoline, (of which there are many derivatives including Edaravone) an industrial laboratory reagent used in synthesis of peptides and making experiments more sensitive. It is used to treat stroke victims. It is the second ALS drug to gain marketing approval in the USA. It slows the progression of ALS by 33% for six months and is effective in 7% of PALS (Data 2016 Source: Mitsubishi Tanabe). It was approved in USA after a small 6 month clinical trial in selected patients in Japan that met a specific exclusion criteria. In 93% of PALS it had no efficacy in earlier trials. A follow-on trial of 12 months duration failed to show any statistical significance of efficacy[1]. Application for approval in Europe was withdrawn in 2019.
Radicava after one year treatment chart
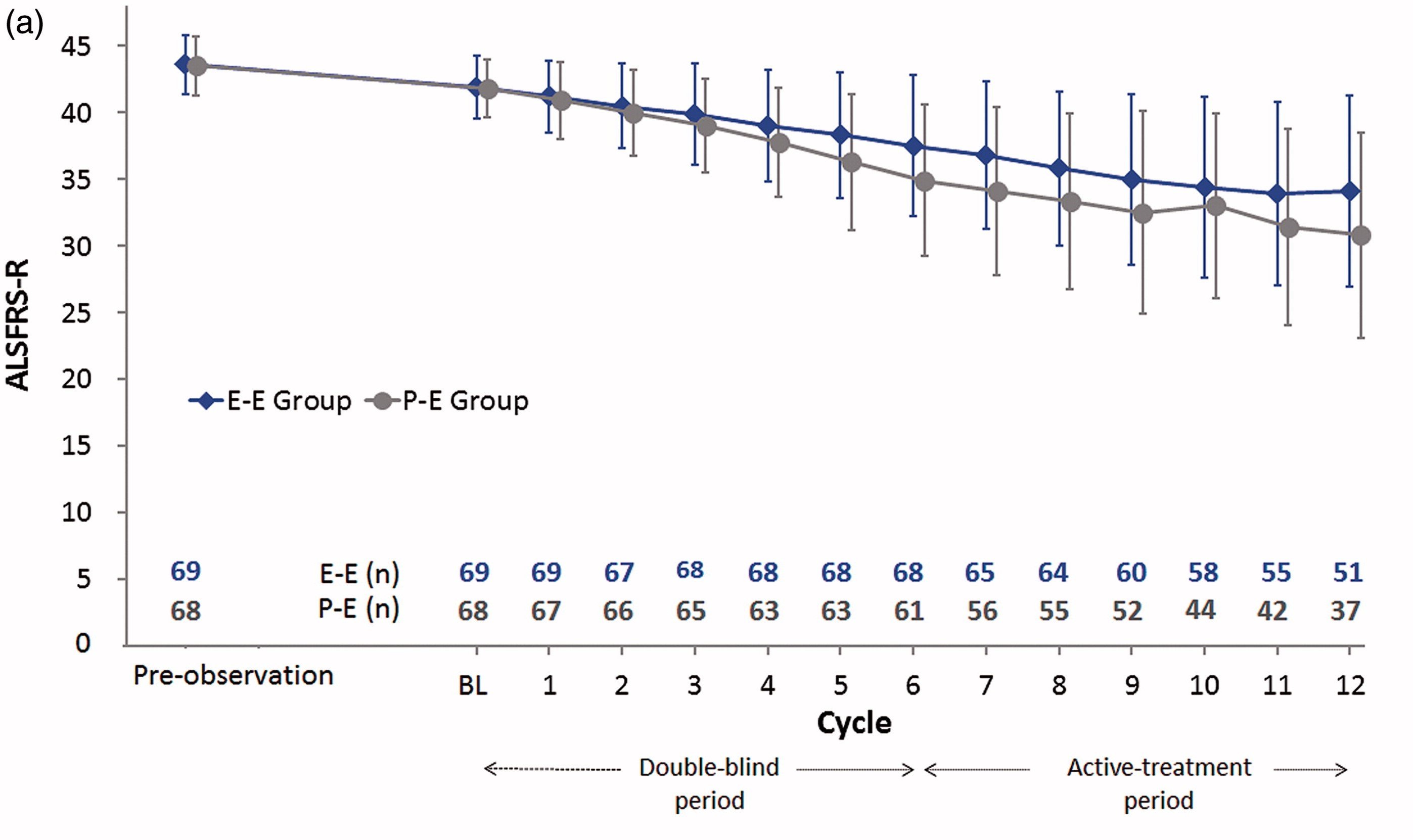
The error bars all overlap. No statistical evidence of ALSFRS efficacy.
1: Source www.tandfonline.com
RCH4
RCH4 is the first and only drug developed specifically for ALS. It slows the progression of PALS by 63% (non-responders included, 70% slowing of progression when the 16% non-responders are excluded) and is effective in 84% of PALS over the whole treated population[1]
RCH4 does not have Regulatory Authority marketing approval as we do not have the millions needed to gain approval. As a charity, PALS treated with RCH4 are not selected or subject to any exclusion criteria as it is done for clinical trials. Everyone asking for help is accepted if possible. Prior careful selection of candidates for clinical trials who are likely to do better, skews efficacy data in favour of the drug being tested in the trial - which can be misleading. RCH4 is provided as a charity, not a clinical trial.
1: Data 2017* Source: Patients own monthly monitoring reports >4,000 datum points
Comparisons
each question is expandable and will be comparing Riluzole, Radicava and RCH4
No cure exists
-
Riluzole: No
-
Radicava: No
-
RCH4: No
-
Riluzole: No / uncertain. However it extends time to tracheostomy by 90 days.
-
Radicava: Yes / uncertain. Effective in 7% of PALS. Theoretically yes but now in doubt. Application withdrawn in Europe in 2019
-
RCH4: Yes / certain. Effective in 85% of PALS. Life expectancy increased by threefold. It has no effect for 15% of those who take it.
-
Riluzole: Perhaps 6%
-
Radicava: 33%
PALS meeting a special criteria [7% of PALS] in 6 months clinical trial in Japan in 2016. The 93% which were non-responders in the previous failed trial were excluded -
RCH4: 70.3%
When the 15% non-responders in the whole treated population from 2014 to 2017 are excluded, i.e., same exclusions as Radicava to allow direct comparison.
-
Riluzole: Licensed Globally
-
Radicava: Licensed in USA & Japan
-
RCH4: Unlicensed
-
Riluzole: Yes
-
Radicava: Yes
-
RCH4: No
-
Riluzole:
Dose form: Usually oral tablet
Dose frequency: Daily
Dose at home: Yes
Clinic visits required: No
-
Radicava:
Dose form: Venous infusion
Dose frequency: 10 days per month
Dose at home: No, unless a PICC catheter is arranged
Clinic visits required: Yes, unless the PICC catheter is arranged
-
RCH4:
Dose form: Muscle injection, using syringe similar to the one for insulin injection**
Dose frequency: Twice weekly typically
Dose at home: Yes
Clinic visits required: No
-
Riluzole: ~ $1,000 [1]
-
Radicava: ~ $12,000 for USA, ~$3,000 for Japan [1]
-
RCH4: No cost / free of charge, provided by this charity if or when sufficient funding is available.
1: source Forbes
-
Riluzole: Clinical trials, data provided by the manufacturer
-
Radicava: Clinical trials, data provided by the manufacturer
-
RCH4: Patient own monthly monitoring reports
-
Riluzole: Yes
-
Radicava: No (as in 2017)
-
RCH4: Yes
-
Riluzole: No
-
Radicava: Yes
-
RCH4: Yes
-
Riluzole: No / Uncertain.
-
Radicava: No - “The mechanism by which Radicava exerts its therapeutic effect in patients with ALS is unknown” - Source FDA package page 6
-
RCH4: Yes - RCH4 is the very first drug specifically made for ALS. A first-in-class treatment evidencing unparalleled safety and efficacy in slowing ALS progression
-
Riluzole: No. Systemic.
-
Radicava: No. Systemic.
-
RCH4: Yes - A specific B-cell sub-set lipid raft receptor
-
Riluzole: Epilepsy, i.e Riluzole is a repurposed drug.
-
Radicava: Stroke victims, ie. Radicava is a re-purposed drug.
-
RCH4: ALS/MND, RCH4 is the first successful drug developed specifically to treat ALS/MND
-
Riluzole: Many thousand of years of patient self dosing
-
Radicava: 6 month clinical trial
-
RCH4: 82 treatment-years of patient self dosing
-
Riluzole: Generally minor or acceptable
-
Radicava: Urine Glucose / Liver
-
RCH4: No relevant side effects reported.
Note: supply of RCH4 got withdrawn for some due to use of suspected counterfeit Radicava and the danger of the side effects due to the counterfeits. The only reports of side effects were from those who were using other counterfeit ALS drugs.
-
Riluzole: unknown
-
Radicava: Yes - Some 93% excluded as they did not meet the required criteria including FVC > 80%, Decline < 1.3, Live independently, etc.
-
RCH4: No, everyone is accepted, subject to drug availability, legal or clinical contra-indication issues or charity lack of funds to treat more PALS
| Evaluation of efficacy → | Major | Moderate | Slight | None | Cannot tell |
|---|---|---|---|---|---|
| Riluzole | 3% | 6% | 8% | 15% | 68% |
| Radicava | 17% | 18% | 20% | 45% | |
| RCH4 | 77% | 18% | 5% |
2020 update
| Evaluation of efficacy → | Major | Moderate | Slight | None | Cannot tell | Number of Reports |
|---|---|---|---|---|---|---|
| Riluzole | 3% | 6% | 8% | 15% | 68% | 1116 |
| Radicava | 1% | 22% | 12% | 16% | 49% | 67 |
| RCH4 | 90% | 6% | 2% | 2% | 162 |